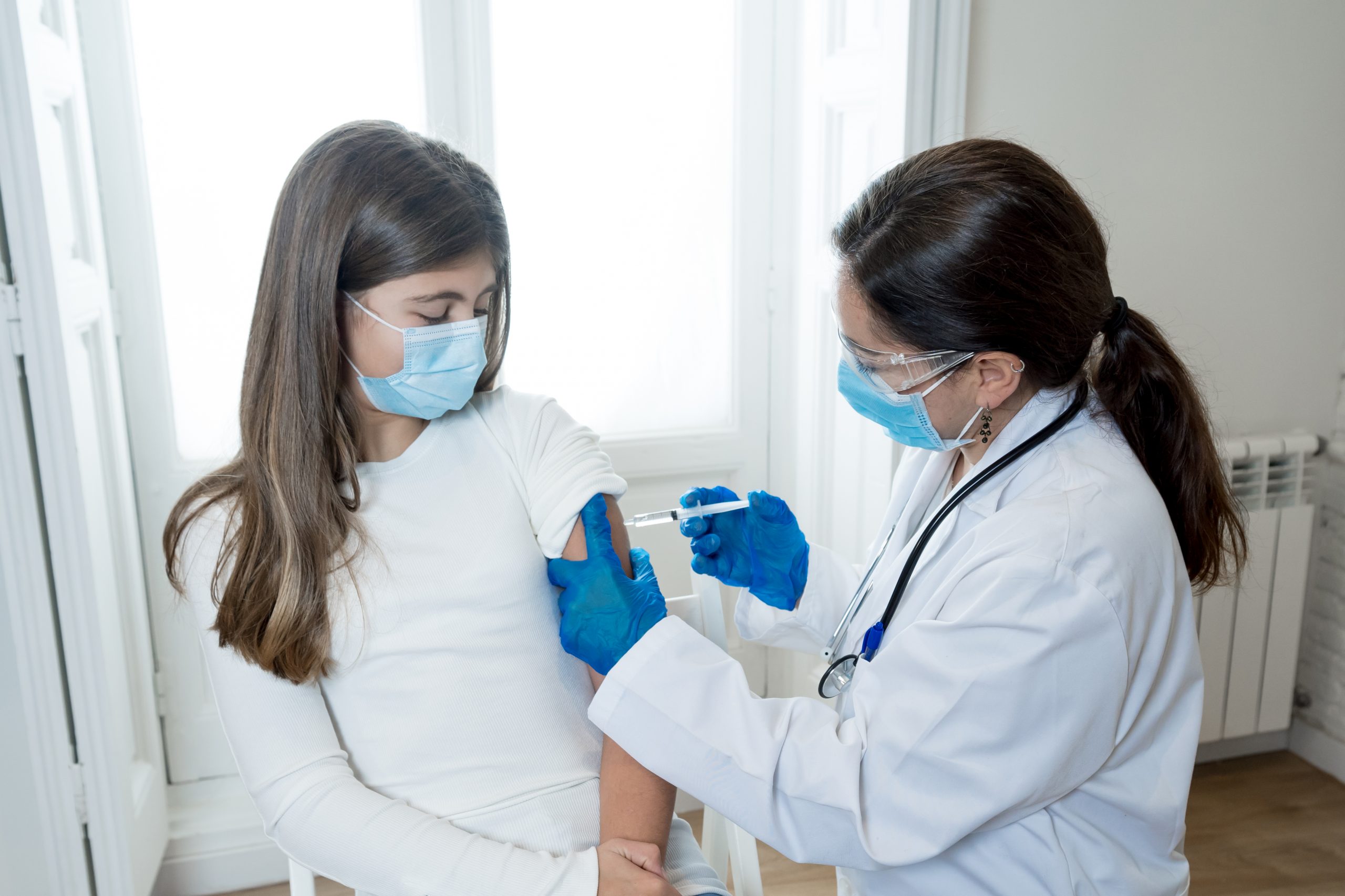
The U.S. Food and Drug Administration has authorized Pfizer’s COVID-19 vaccine for children ages 5 to 11.
On Friday, the FDA issued emergency use authorization that will make the vaccine available to approximately 28 million kids in the United States. Now, the Centers for Disease Control and Prevention must give their approval before the shots can be distributed to children nationwide. The agency will conduct an advisory committee meeting to review the pediatric doses next week and is expected to sign off on their use shortly after.
FDA Commissioner Dr. Janet Woodcock says providing younger children with the COVID-19 vaccine will “bring us closer to returning to a sense of normalcy.”
The FDA emergency use authorization arrived after the Vaccines, and Related Biological Products Advisory Committee voted to support a smaller dose of the Pfizer vaccine for young children.
Nearly 6.3 million children have tested positive for COVID-19 as of October 21st. The CDC says COVID-19 is the eighth-highest killer of kids ages 5 to 11 over the past year.