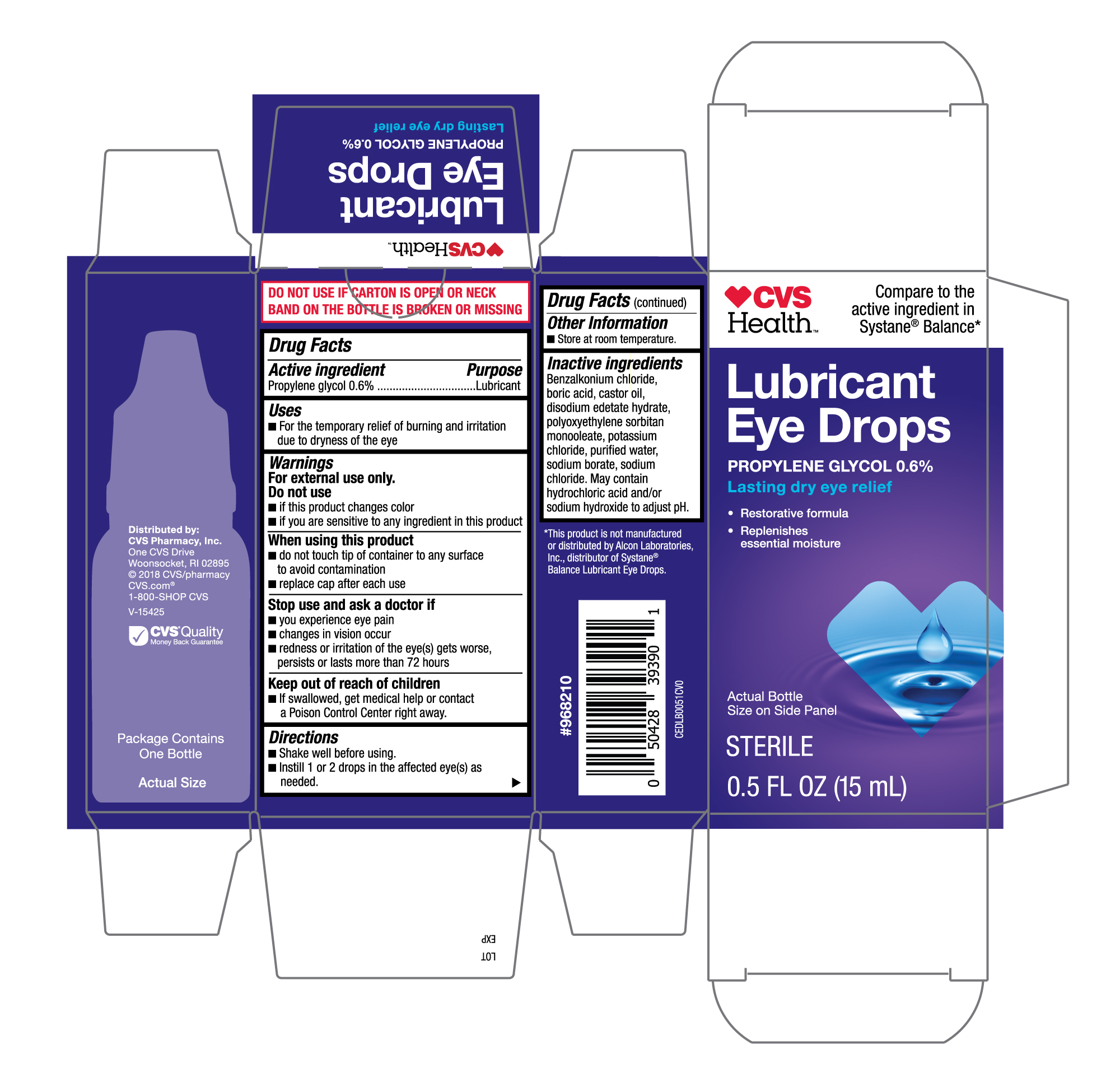
The FDA has flagged over two dozen over-the-counter eye drop products at all major retailers like CVS, Target, and Rite Aid.
According to reports, consumers are being advised of these products because of potential eye infections due to unsanitary manufacturing conditions, which could lead to partial vision loss or blindness.
“These products are intended to be sterile. Ophthalmic drug products pose a potential heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses,” the FDA said in a statement Friday. “FDA recommended the manufacturer of these products recall all lots on October 25, 2023, after agency investigators found insanitary conditions in the manufacturing facility and positive bacterial test results from environmental sampling of critical drug production areas in the facility.”
The FDA identified 26 affected eye drop products sold under various brands like CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up&Up, and Velocity Pharma, with a complete list available on the FDA’s website.
The agency is urging the immediate stoppage of the use of eye drop products and proper disposal via drug take-back sites or following FDA guidelines for trash disposal.
Target, Rite Aid, and CVS are pulling the affected eye drop products from their stores and websites. However, products labeled as Leader, Rugby, and Velocity “may still be available.”
According to ABC, Cardinal Health, the company behind the brands Rugby and Leader, is working to “initiate a recall” of its impacted products.
“FDA notified us of a possible eye infection risk affiliated with some of our Rugby Laboratories and Leader branded eye care products, and immediately upon being notified, we placed all identified impacted eye drop products in our inventory on hold and contacted Velocity Pharma, the supplier of the impacted eye drop products,” Cardinal Health said in a statement. “We are in the process of working with Velocity Pharma and FDA to initiate a recall of all impacted Rugby Laboratories and Cardinal Health Leader branded eye drop products to further safeguard public health and safety.”
The statement continued, “We take FDA’s consumer notification regarding the risk of using impacted Rugby Laboratories and Leader-branded eye drop products very seriously. We are working with Velocity Pharma, the supplier of the impacted eye drop products, to gain additional insight regarding the unsanitary conditions identified by the FDA at the manufacturing facility.”
Rite Aid told ABC, “Due to safety concerns identified by the FDA, we are removing the applicable Rite Aid-branded products from our store shelves.”
Additionally, CVS stated that they would give a full refund to anyone who purchased an impacted product.