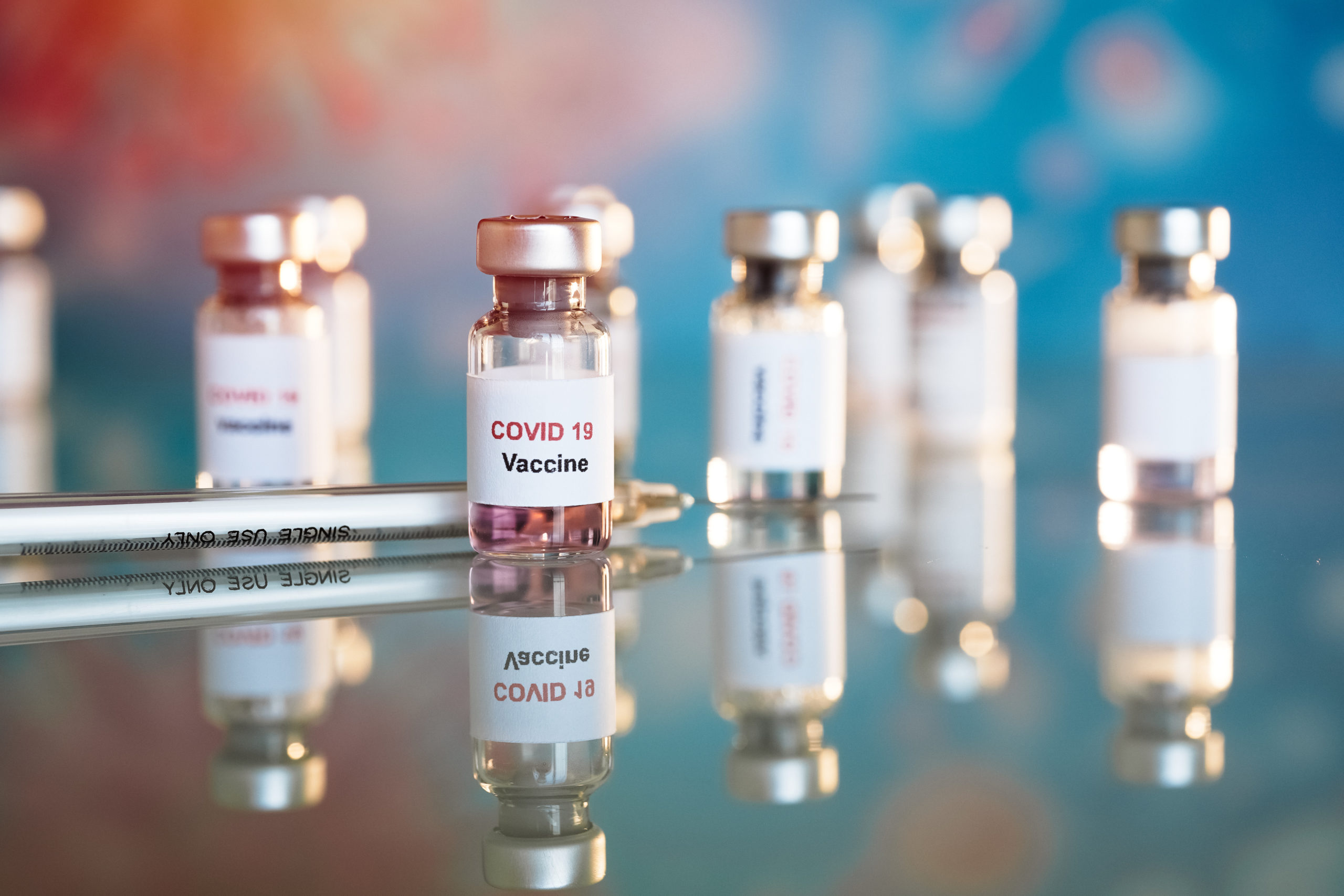
An advisory committee of the Food and Drug Administration unanimously recommended that people ages 65 and older and other vulnerable Americans should get Moderna’s COVID-19 booster shot.
CNBC News reported that the vote was crucial for the country to administer third shots to some of the 69 million people who originally received the vaccine.
The FDA’s Vaccines and Related Biological Products Advisory Committee decision would bring guidelines for Moderna in line with third shots of the Pfizer-BioNTech vaccine. Less than a month ago, those shots were authorized to include the elderly, those with underlying medical conditions, and people who work or live in high-risk areas.
A final FDA decision on Moderna boosters could come within days. The next step would be for the CDC vaccine advisory committee to vote on the FDA’s proposal. If it recommends approval and the CDC endorses it, booster shots could immediately be distributed to eligible Americans who completed their immunizations at least six months ago.
Booster shots have been a topic of conversation among scientists considering many people have yet received even one dose of a vaccine. The World Health Organization suggests wealthy countries should hold off on distributing boosters, and some scientists aren’t convinced that Americans need boosters right now.
The Biden administration is hopeful that offering the U.S. population additional doses will ensure long-term and durable protection against severe disease, hospitalization, and death as the fast-moving delta variant continues to spread.

![Kelly Price Slams Promoter for Failing to Pay When She Arrived to Perform Friday [Video] - Baller Alert Kelly Price Slams Promoter for Failing to Pay When She Arrived to Perform Friday [Video]](https://balleralert.com/wp-content/uploads/2021/09/Screen-Shot-2021-09-24-at-9.29.03-PM-e1733586655504-75x75.png)
